Back to your search result
Hazards
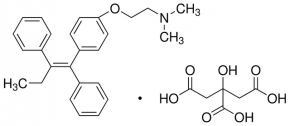
Structure formula
Contents
Miscellaneous
Certificate of Analysis (specimen)
Reference Material CoA specimen: for the current lot, please contact your customer service representative at info@labmix24.com
Product data sheet
View all available product details e.g. description, analytes/parameters, CAS Number, concentrations/values, sales unit/product format, method, source, transport information
US Pharmacopeia Standards set a global benchmark, enabling efficient, reliable, and cost-effective development and quality control of pharmaceuticals.
Official USP Reference Standards are highly characterized physical specimens intended for quality control use when conducting assays and tests for medicines as described in the USP-NF. When you conduct tests and assays required by the USP-NF using the USP reference standards specified, the results can be considered conclusive.
As a USP-Authorized Distributor, we offer the following products and services:
- USP Reference Standards
- USP Pharmaceutical Analytical Impurities
- USP Training and educational programs